 |
|

Improving Human and Animal Health With Protein Engineering.
|
|
|
|
Trophogen has a unique and very broad platform technology to develop patented long-acting, much more potent & effective, and totally safe non-immunogenic human and animal superagonist analogs of glycoprotein hormones and growth factors. Our products address multi-billion dollar markets in infertility, elective egg banking, and untreatable & aggressive cancers. The first 3 human products of this very broad platform, FSH, TSH, and VEGF superagonist analogs plus Nanotechnology, are fully developed and ready for IND and Clinical Trials. Trophogen’s BioSuperior platform technologies will supercede all current BioBetters, BioSimilars, and current market drugs in their respective imaging, diagnostic and therapeutic space.
|
|
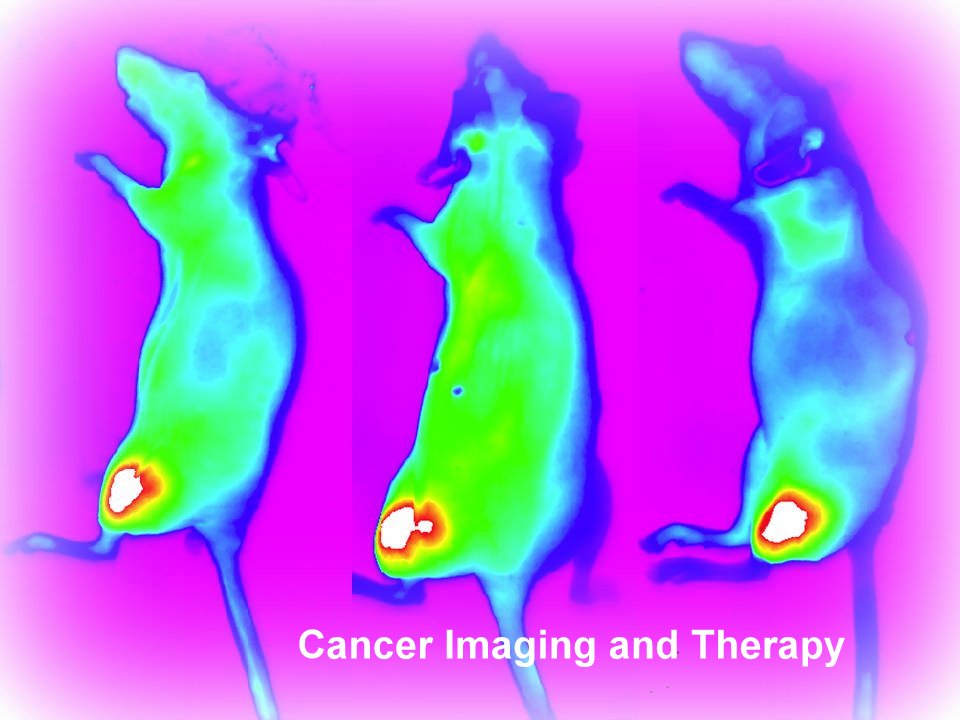
Trophogen, Inc. Awarded $756,000 for Second Year of Phase 2 NIH/NCI Fast Track SBIR Grant for Development of Novel Human VEGF Analogs for Targeted Imaging of Undifferentiated Thyroid Cancer.
July 7th, 2015 - FOR IMMEDIATE RLEASE Rockville, MD
Trophogen, Inc. today announced that it was awarded the second year of its Phase 2 Fast Track component of $756,000 for a Small Business Innovation Research (SBIR) Award from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) to develop novel VEGF analogs for targeted imaging of undifferentiated thyroid cancer.
|
| |
Trophogen, Inc. Announces Signing of Exclusive Global Veterinary License and Purchase Agreement With Zoetis, Inc. for Recombinant Long- Acting and Superactive Bovine Follicle- Stimulating Hormone (rbFSH) Analog for Superovulation in Cows.
June 2nd, 2015 - FOR IMMEDIATE RLEASE Rockville, MD
Trophogen, Inc. today announced that the company has completed a licensing and purchase agreement granting Zoetis Inc., the leading global animal health company, an exclusive license to develop Trophogen’s recombinant modified bovine Follicle- Stimulating Hormone (rbFSH) analog in the field of animal health reproduction.
|
|
|